-
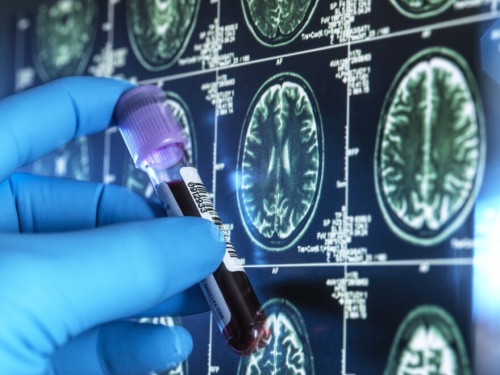
We are excited to share that CEOi’s Blood-Based Biomarker Workgroup publication, “The Global CEO Initiative on Alzheimer’s Disease performance recommendations for blood-based biomarker tests,” is now released in Nature Reviews Neurology. Click here to read the publication.

The Global CEO Initiative on Alzheimer’s Disease (CEOi), founded in 2013, is an organization of private-sector leaders who have joined together to provide business leadership in the fight against Alzheimer’s. The CEOi believes that, during this era of aging populations, it will take visionary, coordinated, goal-oriented leadership of public and private leaders working together to solve our greatest challenges. It is convened by UsAgainstAlzheimer’s.
Our members and collaborators
Blood-Based Biomarker Workgroup


In this episode, current and former regulatory officials take a page from the COVID-19 playbook, highlighting the vital need for transparent communication and cooperation across all stakeholders. The opportunity when done right?: The advancement of incremental innovations that can eventually transform a devastating disease into a manageable condition.