
A network of UsAgainstAlzheimer’s.
Join the Network
About Us
ResearchersAgainstAlzheimer’s, a network of UsAgainstAlzheimer's, is a global coalition of over 500 leading Alzheimer’s researchers that advocates for research funding and policy reform to support our goal of preventing and treating Alzheimer’s disease. Given the challenges facing Alzheimer’s patients, caregivers, and healthcare professionals today, the Researchers network considers the development of a coordinated global research strategy critical.
If government, private industry, and the research community unite with a common goal and purpose, backed with the necessary leadership, resources, and incentives, the power of scientific research can be unleashed to deliver new cures and prevent and treat Alzheimer’s disease.
Beliefs
We have heard some say that Alzheimer’s disease is an inevitable part of aging. Others have claimed that it simply cannot be prevented or effectively treated anytime soon. Still others believe that we simply cannot afford to do what it would take to stop the disease. They are all wrong.
As men and women of science, we are united by a simple but bold belief: It is possible to prevent and effectively treat Alzheimer’s disease within our lifetimes, but only if our nation does what is necessary:
- Set an aggressive goal of stopping Alzheimer’s disease by 2025, because it will focus the energies of the research community;
- Invest significant resources in Alzheimer’s research and innovation, because our commitment to cures must match the scale of the challenge;
- Institute reforms to accelerate the drug pipeline and deliver therapies to patients faster, because the ultimate goal of research is to bring help to those afflicted, and to prevent the disease from afflicting future generations.
If government, private industry, and the research community unite with a common goal and purpose backed with the necessary leadership, resources, and incentives, the power of scientific research can be unleashed to unlock new possibilities, to deliver new cures, and to prevent and treat Alzheimer’s disease.
Founding Members
|
Paul Aisen, MD Founding Director of the University of Southern California Alzheimer's Therapeutic Research Institute |
Howard Fillit, MD Executive Director, Alzheimer's Drug Discovery Foundation |
Reisa Sperling, MD Director, Center for Alzheimer's Research and Treatment, Brigham and Women's Hospital, Massachusetts General Hospital, Harvard Medical School |
|
Marilyn Albert, PhD Director of the Division of Cognitive Neuroscience in the Department of Neurology at Johns Hopkins University School of Medicine, and Director of the Johns Hopkins Alzheimer's Disease Research Center |
David Holtzman, MD Chair, Department of Neurology, Washington University, St. Louis |
Rudy Tanzi, PhD Director, Genetics and Aging Research Unit, Mass General Institute for Neurodegenerative Disease, and Joseph P. and Rose F. Kennedy Professor of Neurology, Harvard Medical School |
|
David Bennett, MD Robert C. Borwell Professor of Neurological Sciences and Director of the Rush Alzheimer's Disease Center |
Takeshi Iwatsubo, MD, PhD* Professor of Neuropathology at the School of Medicine, University of Tokyo |
John Trojanowski, MD, PhD Co-Director, Center for Neurodegenerative Disease Research, and Director of the Alzheimer's Disease Core Center of the University of Pennsylvania |
|
Jeff Cummings, MD Director of the Cleveland Clinic Lou Ruvo Center for Brain Health |
Bruce Lamb, PhD Executive Director, Stark Neurosciences Research Institute, Indiana University |
Michael Weiner, MD Professor in Residence in Radiology and Biomedical Imaging, Medicine, Psychiatry, and Neurology at the University of California, San Francisco, and Principle Investigator of the Alzheimer's Disease Neuroimaging Initiative |
|
Steve DeKosky, MD Aerts-Cosper Professor of Alzheimer's Research at the University of Florida College of Medicine |
Simone Lovestone, PhD* Professor of Translational Neuroscience, Department of Psychiatry-Medical Sciences Division, University of Oxford |
Ron Peterson, MD, PhD Director, Mayo Alzheimer's Disease Research Center |
|
Rachelle Doody, MD, PhD Global Head of Neurodegeneration in Product Development, Neuroscience at Roche Pharmaceutical Company |
David Morgan, PhD Professor of Translational Sciences, Director of the Alzheimer's Alliance, Michigan State University |
Affiliations are for identification purposes only and do not necessarily represent the endorsement of the affiliated institution.
* indicates the representative is serving as a strategic advisor to ResearchersAgainstAlzheimer’s
Member Expectations
Members of the network will be expected to
- Concur with and add their name to the ResearchersAgainstAlzheimer’s Statement of Beliefs;
- Act as an advocate for Alzheimer’s research in their home country and with international organizations;
- Attend ResearchersAgainstAlzheimer’s events when schedule and availability allow;
- Provide scientific expertise and insights to inform policy maker outreach as schedule permits; and
- Share with ResearchersAgainstAlzheimer's their ideas on what a national and global public policy strategy to increase research in Alzheimer’s should be
Latest Updates
Learn about Clinical Trials
Featured Reports
Each year, ResearchersAgainstAlzheimer’s (RA2) releases a report on Alzheimer’s drugs in late-stage clinical development and other related topics. Check out the latest reports.
 RA2’s Phase 2 & 3 Pipeline Poster (July 2019)
RA2’s Phase 2 & 3 Pipeline Poster (July 2019)
The 2019 Alzheimer's drug pipeline report showcases an array of approaches in development to treat the disease, including novel vaccines, gene therapies and interventions designed to target bacterial and viral infections associated with Alzheimer's.
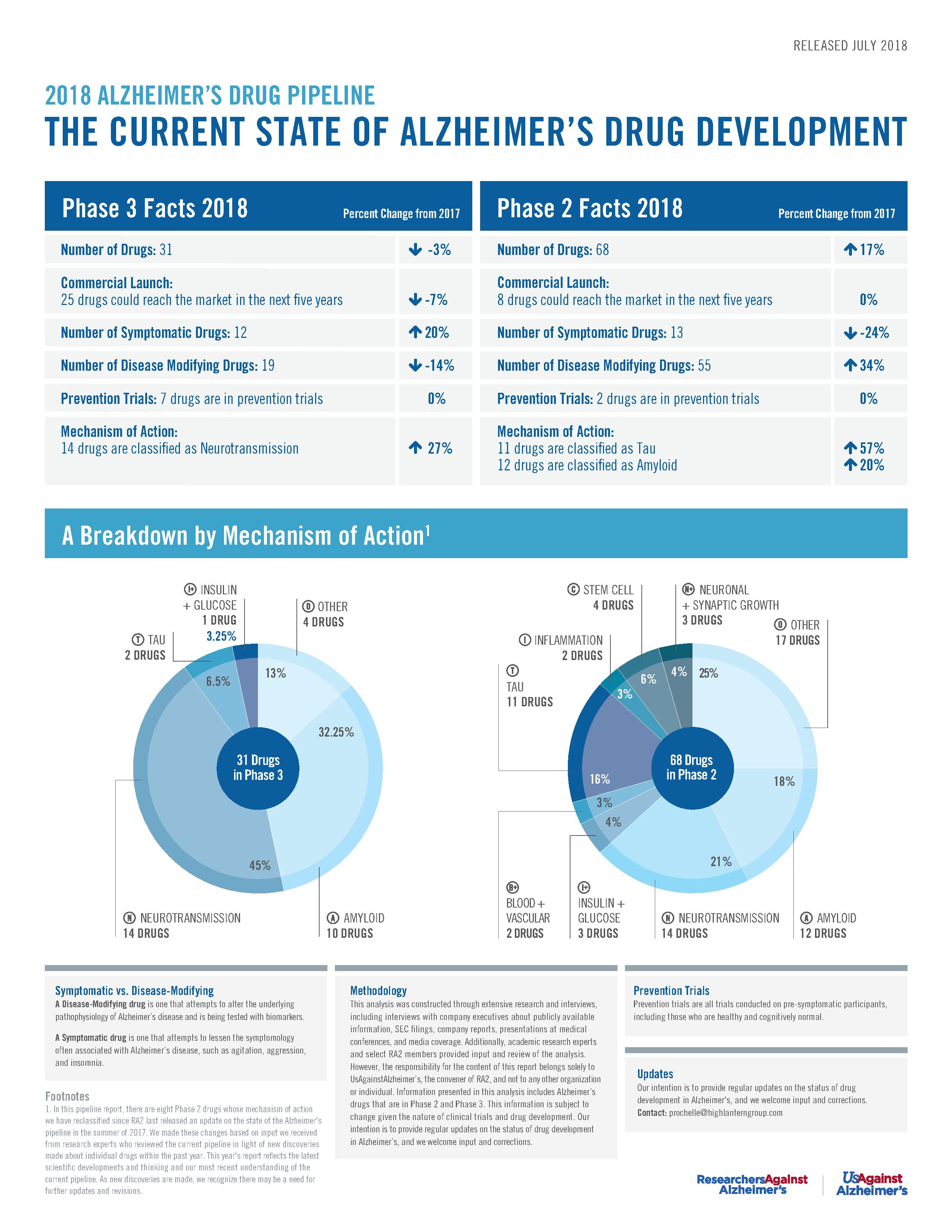 RA2’s Phase 2 & 3 Pipeline Poster (July 2018)
RA2’s Phase 2 & 3 Pipeline Poster (July 2018)
ResearchersAgainstAlzheimer’s calls on governments, industry, entrepreneurs, and world leaders to improve the healthcare system to respond to today's patients and caregivers and accommodate future Alzheimer’s disease treatments in its latest drug pipeline analysis.
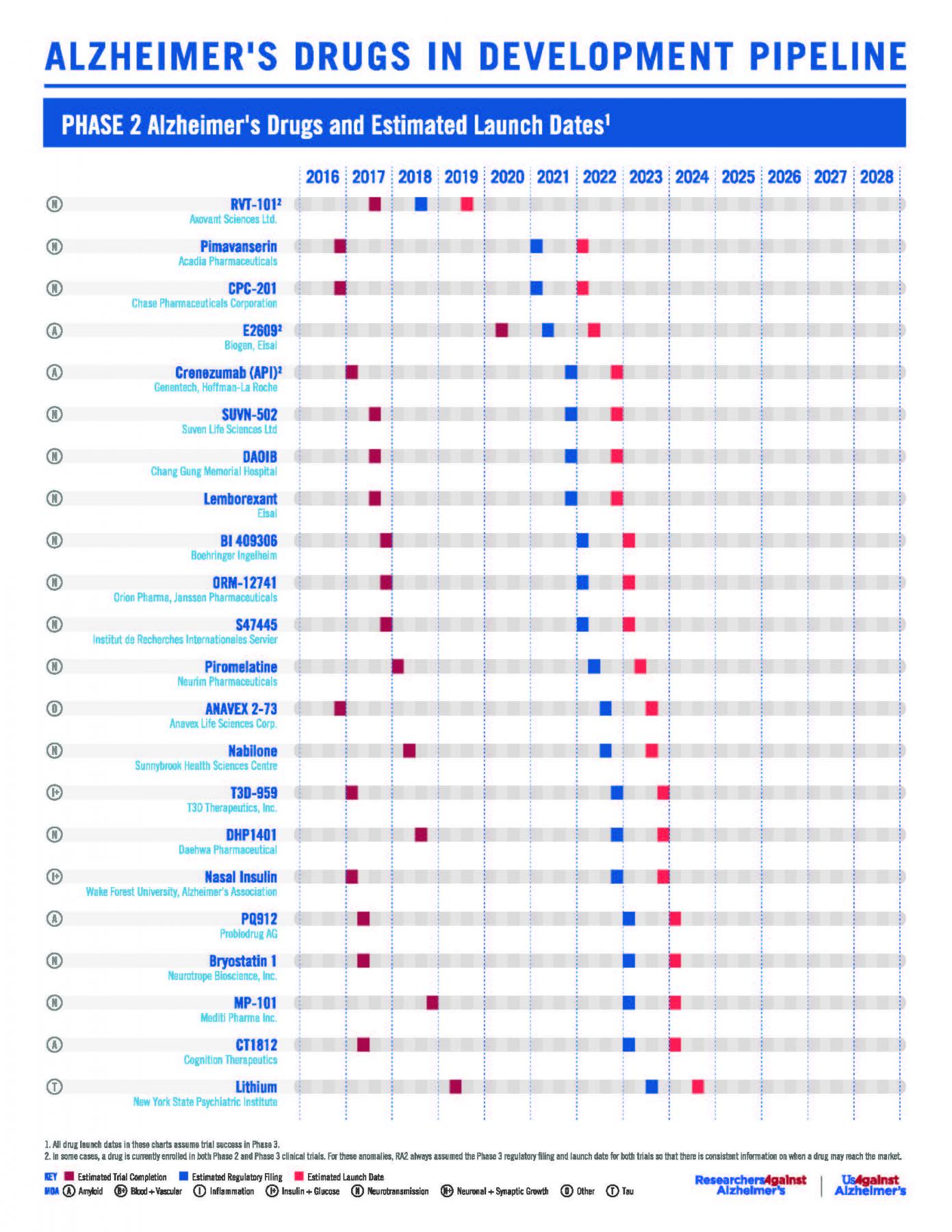 RA2’s Phase 2 & 3 Pipeline Poster (July 2017)
RA2’s Phase 2 & 3 Pipeline Poster (July 2017)
Twenty-seven Alzheimer’s drugs in Phase III clinical trials and eight drugs in Phase II clinical trials may launch in the next five years, according to a revised Alzheimer’s pipeline analysis presented at the Alzheimer’s Association International Conference (AAIC) by ResearchersAgainstAlzheimer’s.
 Single Endpoint for New Drug Approvals for Alzheimer’s Disease (May 2017)
Single Endpoint for New Drug Approvals for Alzheimer’s Disease (May 2017)
The FDA's approach to approving new medicines for Alzheimer’s disease since the 1990s has been to require a proven benefit on two independent primary endpoints of cognition and function, although this policy has not been formalized in the FDA rules or finalized FDA guidance. The FDA should clarify for the field that a clinically meaningful benefit on a single primary endpoint of cognition or function is a sufficient basis for a New Drug Application filing.
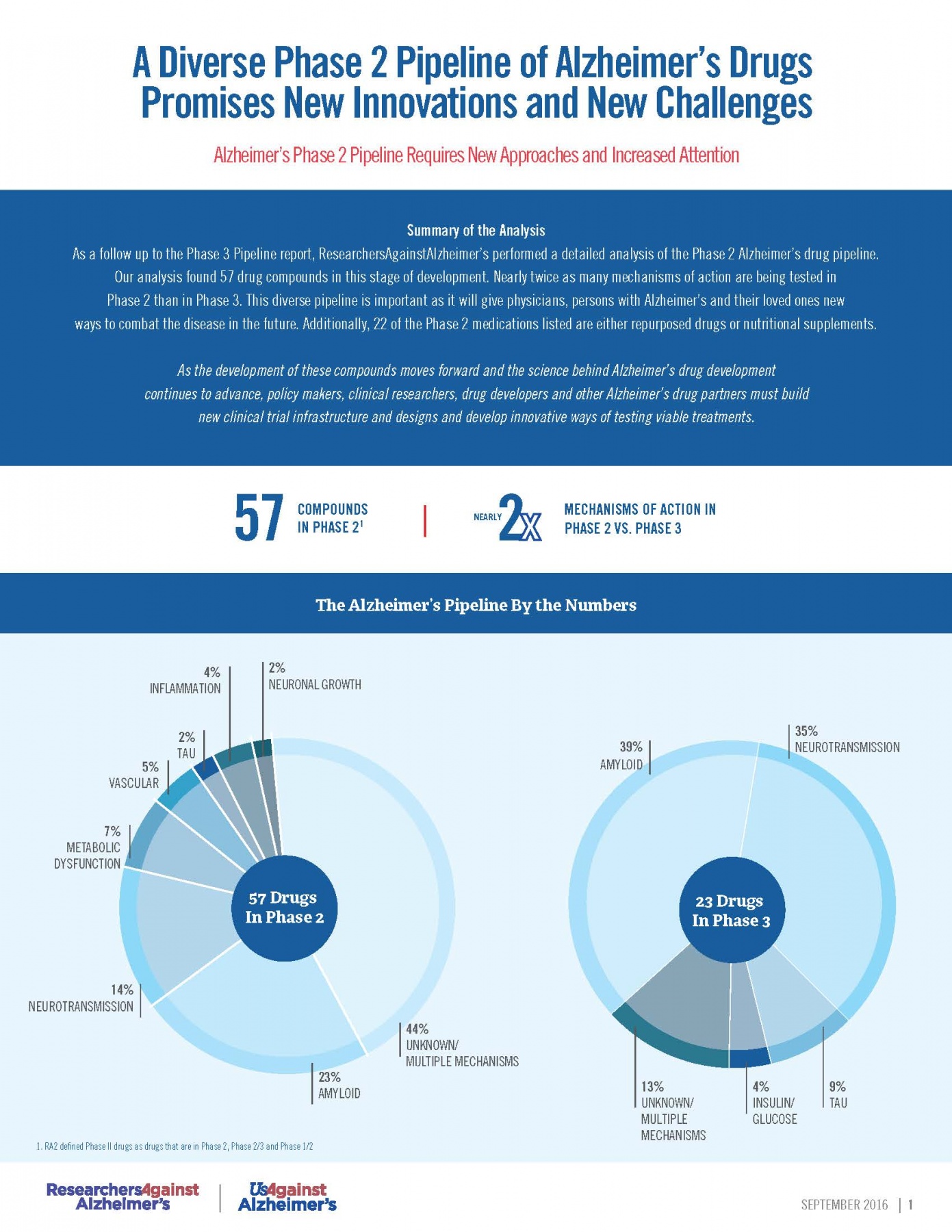 RA2’s Phase 2 Pipeline Analysis (September 2016)
RA2’s Phase 2 Pipeline Analysis (September 2016)
A new analysis of the Phase II Alzheimer’s drug pipeline, conducted by RA2, revealed 57 new Alzheimer’s drugs. According to the analysis, nearly twice as many mechanisms of action are being tested in Phase II as compared to Phase III clinical trials.
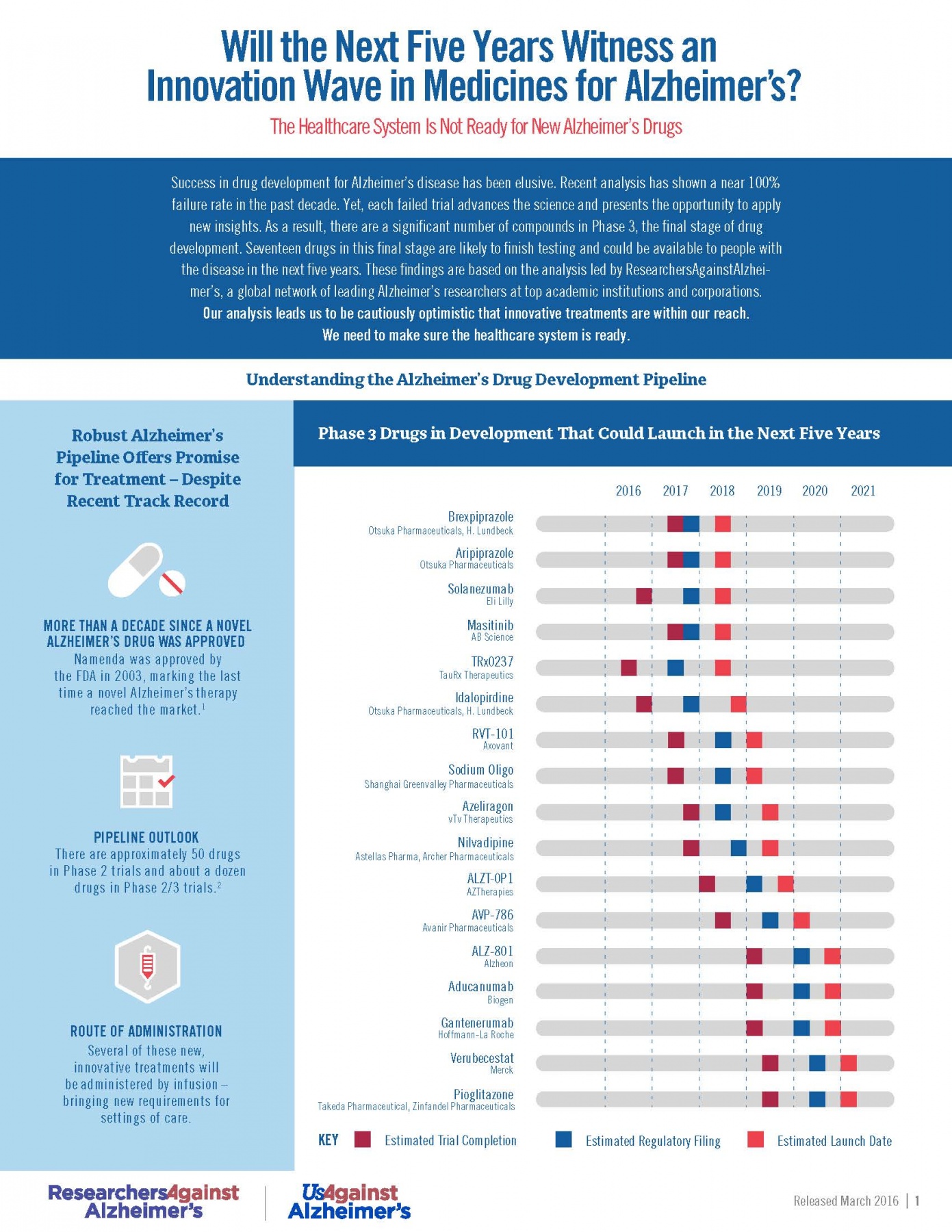 RA2’s Phase 3 Pipeline Analysis (March 2016)
RA2’s Phase 3 Pipeline Analysis (March 2016)
Today’s Alzheimer’s disease drug pipeline, marred by more than a decade of high failure rates and public underinvestment, is offering near-term promise with 17 drugs on pace to launch in the next five years, according to a recent analysis conducted by ResearchersAgainstAlzheimer’s.
Visit the Action Center to help stop Alzheimer's.
Help Us Stop Alzheimer’s: Donate Today
We can stop Alzheimer’s but not without you. Thanks to your support, we’re able to continue raising awareness and fighting to bring an end to this debilitating disease: 100% of donations received by UsAgainstAlzheimer’s are devoted to our efforts to stop Alzheimer’s.